Why Is Fluorine More Electronegative Than Chlorine
The order of polarity is the same as the order of electronegativity of the 2nd atom. It is the lightest halogen.
Electron affinity refers to the energy released when an electron is accepted by a neutral atom.
. Therefore atoms closer to fluorine will be more electronegative. Though fluorine is the most electronegative atom it has lower electron affinity than chlorineIt is due to their compact atomic size which propels it high electron density inside the atomSo the incoming electron somehow feels a repulsion which leads to lower value of affinity. Reactivity is an elements ability to gain an electronSo the better it is at stealing electrons the more reactive it will be.
Therefore Chlorine has a higher electron affinity than Fluorine. A The fluorine atom is smaller than the chlorine atom and there is less shielding from other shells of electrons. Thus it is more strongly attracted to the delta positive hydrogens on a water molecule.
The closer it gets to being full we know that electronegativity increases. The thing that makes fluorine so reactive is its electronegativity. Why is fluorine F2 more reactive than chlorine Cl2.
With Fluorine only needing one more electron to be complete. This is because the valencebonding electrons are closer to the nucleus in Fluorine than they are Chlorine and others and thus more strongly attracted. Fluorine is the most electronegative atom.
Answer 1 of 7. Fluorine is a smaller atom so when it forms a covalent bond the shared electrons are closer to the nucleus and there is less shielding. Similarly why is fluorine more reactive than carbon.
Although Chlorine has a higher electron affinity not by very much though Fluorine is a much stronger oxidant under standard conditions this is due to the anomalously weak F-F bond high hydration enthalpy due to small size and charge density of F and high lattice energy these factors more than make up for the slightly lower electron affinity compared to Chlorine. Hope this helps you. Oxygen is more electronegative than chlorine because of the following reasons.
The big battle for second place is interesting. KL where as chlorine has 3 shell ie. Fluorine is a pale yellow diatomic highly corrosive flammable gas with a pungent odor.
It is because fluorine has the Highest electronegativity and the size of atom is small so Fluorine is much non metallic than Chlorine. Fluorine is more electronegative than chlorine because fluorine is smaller and has its electrons closer to the positively charged nucleus. Why chlorine is more electronegative than fluorine.
- Fluorine has the maximum electronegativity. See full answer below. Why is fluorine more electronegative than chlorine Class 11.
A The fluorine atom is smaller than the chlorine atom and there is less shielding from other shells of electrons. B The fluoride ion is smaller than the chloride ion giving it a larger charge density. But we know Oxygen wins out by a slim margin.
Fluorine is the most reactive and the most electronegative of all the elements. Fluorine is more electronegative than chlorine but electron affinity of fluorine is less than chlorine. Since halogens are more electronegative than carbon and also possesses lone pair electrons therefore they exert both -I and R effects.
Therefore since fluorine has a higher electronegatvity than chlorine fluorine is more reactive. Why is fluorine more electronegative. It reacts violently with water to produce oxygen and the extremely corrosive hydrofluoric acid.
Thus the bonding pair of electrons are more attracted to the positive nucleus. What charge is fluorine-1. Fg e- ----- F-g deltaHo -3280 kJmol The electron cloud of F is more dense than that of Cl because Cl is larger in size.
Fluorine is more electronegative than chlorine even then p-flurobenzoic acid is weaker acid than p-chlorobenzoic acid explain. Since fluorine has a very small atomic radius it exerts a repulsive force on any incoming electron. Now in F the lone pair of electrons are present in 2p-orbitals but in Cl they are present in 3p-orbitals.
A The fluorine atom is smaller than the chlorine atom and there is less shielding from other shells of electrons. The reason states that Fluorine has higher electron affinity than Chlorine. As we go down a group the atomic radii of the atom increases even though the nuclear charge also increases the atomic radii increases to a greater extent which practically cancels out the effect of the increased nuclear charge.
Therefore fluorine more electronegative than chlorine. The electron affinity of the fluorine is less than chlorine because the size of fluorine is too small as size decreases from left to right inside period whereas chlorine has a larger size to accommodate electrons hence electron affinity of chlorine is more than fluorine. Oxygen only needs 2 electrons in its valence shell.
Thus the bonding pair of electrons are more attracted to the positive nucleus. Chlorine only needs one but in its 3rd shell. F has greater tendency than Cl to attract the shared pair of electrons of a covalent bond.
A fluorine atom in the gas phase for example gives off energy when it gains an electron to form a fluoride ion. Good Luck. In this regard why is fluorine so reactive.
Chlorine is below fluorine and has a new shell of valence electrons is added to it. What is more reatice fluorine. Fluorine is most electronegative thus it is most reactive.
Oxygen is placed towards the left side of fluorine so has one electron less than fluorine. Since fluorine is smaller than chlorine the attractive forces between the nucleus and the outermost shell or the Valence shell is more stronger as compared to that in chlorine and hence Fluorine is more electronegative than Chlorine. As we know thatchlorine atom has more electrons and protons than florine so chlorine has to be more electro negative than of florine but it is wrong because florine has 2 shell ie.
Why is fluorine special. Electron affinity of chlorine is more means electron can be added into gaseous chlorine atom more easily in chlorine than fluorine. Explain why fluorine is more electronegative than chlorine.

In Group 17 Of The Periodic Table Fluorine Is More Reactive Than Chlorine And Chlorine Is Less Youtube
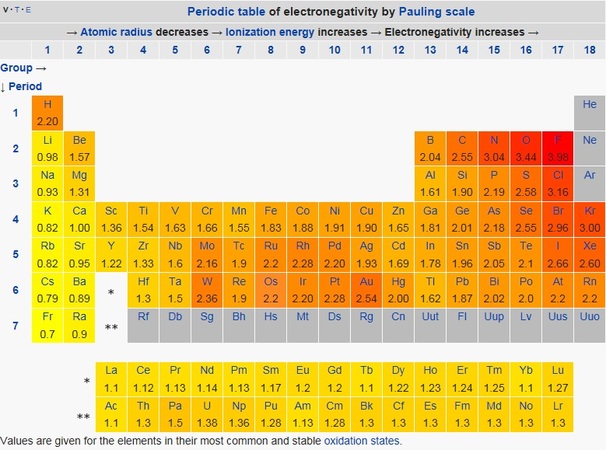
Science Skool Electronegativity And Polarity
Fluorine Is More Electronegative Than Chlorine Even Then P Fluorobenzoic Acid Is Aweaker Base Than P Chlorobenzoic Acid Snapsolve

Fluorine Has Lower Electron Affinity Than Chlorine Because Of Youtube

Fluorine Is More Electronegative Than Chlorine Is The Statement True
How To Predict The Relative Electronegativity Of An Atom

Fluorine Is More Electronegative Than Chlorine Is The Statement True Youtube
Which Is More Electronegative Bromine Or Iodine Quora

Solved Fluorine Is Less Deactivating Than Chlorine In The Chegg Com

Statement 1 Fluorine Is More Electronegative Than Chlorine Statement 2 Fluorine Is Smaller In S Youtube
Is Chlorine More Electronegative Than Flourine Quora
Is Chlorine More Electronegative Than Flourine Quora
Which Element Is More Electronegative Chemistry Community
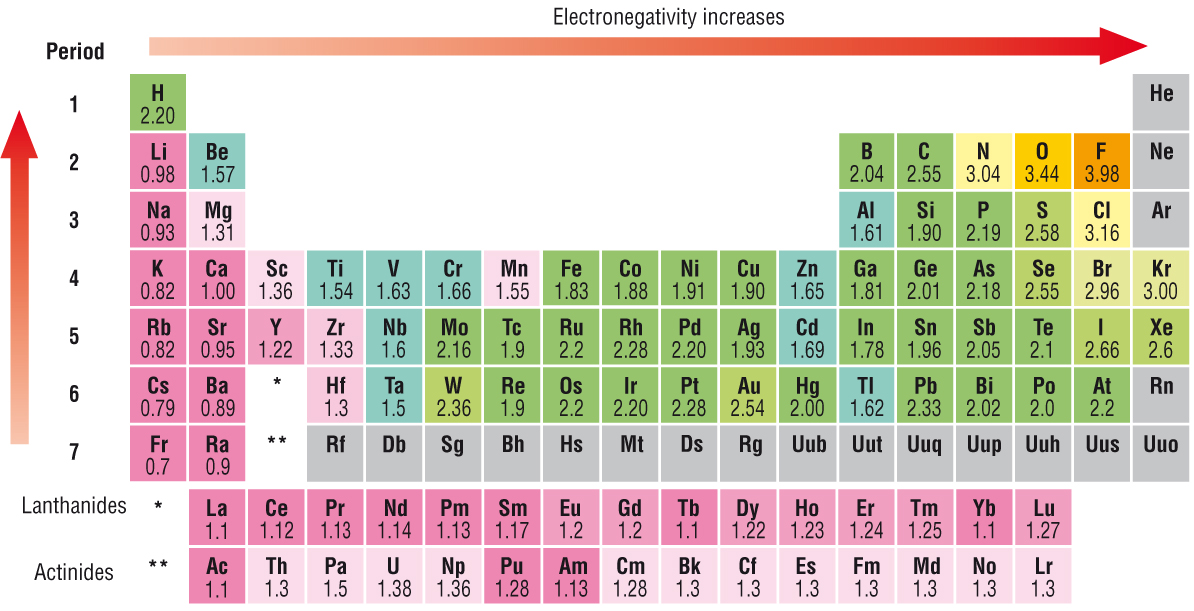
What Would Cause An Atom To Have A Low Electronegativity Value Socratic

Welcome To Chem Zipper Com Fluorine Is More Electronegative Than Chlorine Even Then P Flurobenzoic Acid Is Weaker Acid Than P Chlorobenzoic Acid Explain

Why Is O More Electronegative Than Cl And How Quora

Fluorine Is More Electronegative Than Chlorine Is The Statement True



Comments
Post a Comment